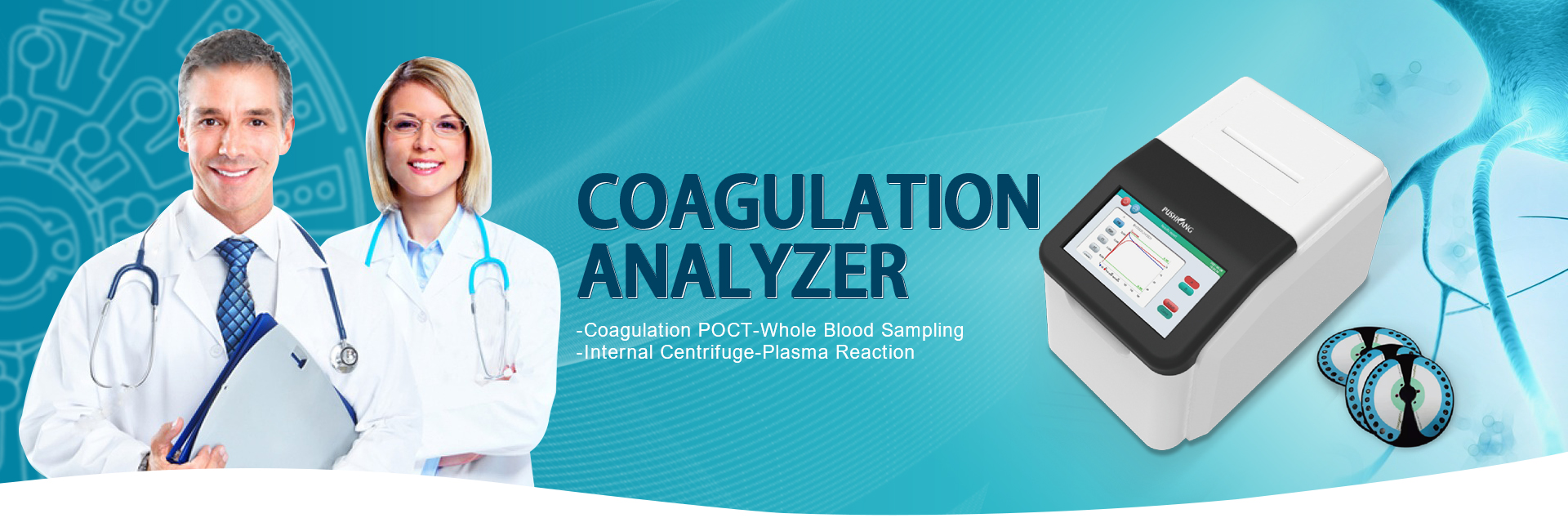
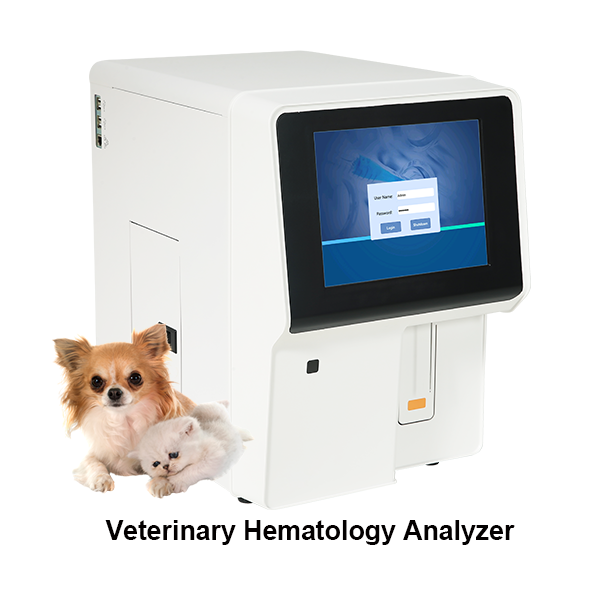
FDA IVD Clearance: Ensuring Compliance for In Vitro Diagnostic Devices
Introducing the innovative FDA IVD product from Zhejiang Pushkang Biotechnology Co., Ltd., a leading manufacturer in China. Our company is proud to offer this cutting-edge in vitro diagnostic product that has been meticulously developed to meet the highest standards of quality and accuracy. Our commitment to research and development has resulted in a product that delivers reliable and precise results, making it an essential tool for healthcare professionals. This FDA IVD product is the result of our company's dedication to providing advanced diagnostic solutions to improve patient care. With our state-of-the-art technology and rigorous testing protocols, customers can trust in the performance and effectiveness of our products. We are confident that this FDA IVD product will set a new standard in the industry and provide valuable insights for medical professionals. Choose Zhejiang Pushkang Biotechnology Co., Ltd. as your trusted partner for high-quality in vitro diagnostic products. Our commitment to excellence and innovation ensures that our FDA IVD product is the ideal choice for your diagnostic needs.
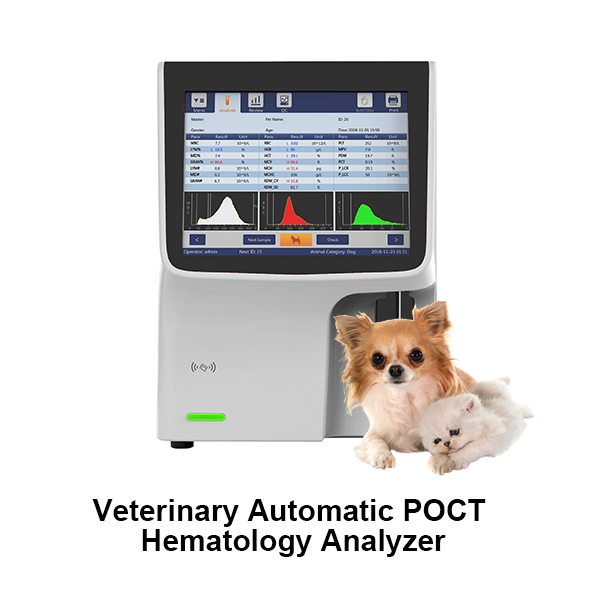
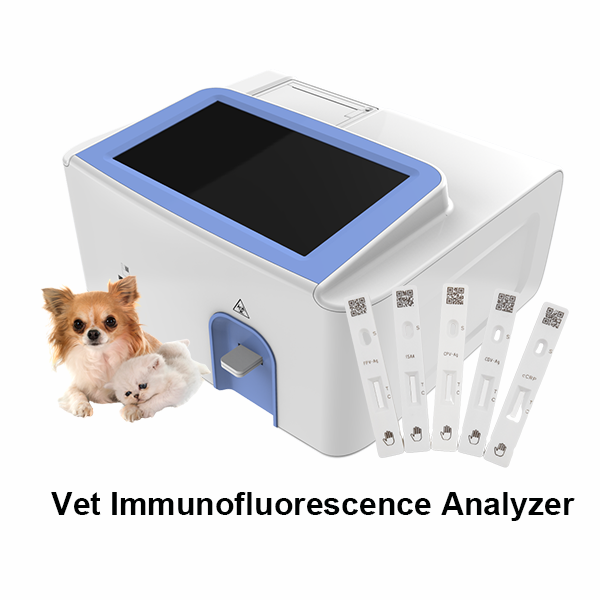
Related Products